Strukturbiologie von Elementzyklen
Forschungsgruppenleiter: Thomas Barends
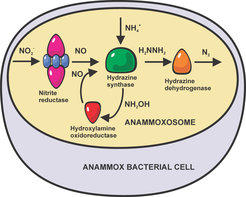
The discovery of anammox bacteria in the 1990's has dramatically changed our understanding of the global nitrogen cycle. These bacteria perform ANaerobic AMMonium OXidation, combining ammonium with nitrite into molecular dinitrogen (N2) and water, yielding energy for the cell. The anammox process is responsible for up to 10% of the global nitrogen cycle, and in some ecosystems contributes up to 50% to the total N2 production. Biochemical studies, mainly by the group of Mike Jetten and coworkers in Nijmegen, have identified the enzymes involved, and showed that the central step in this process is the combination of nitric oxide and ammonia into the extremely unusual, highly toxic and reactive intermediate hydrazine.
In collaboration with the microbiology department of Prof. Dr. Mike Jetten of the Radboud University in Nijmegen, we have determined the structures of key enzymes in this process, with a view to elucidating the mechanism of anaerobic ammonium oxidation. Importantly, we have determined the structure of the enigmatic hydrazine synthase, from which we proposed a mechanism for the biological synthesis of hydrazine[1]. The structure of the Kuenenia stuttgartiensis hydroxylamine oxidoreductase has allowed us to propose a novel mechanism for biological nitric oxide generation [2,3], and the structure of a putative NO-producing enzyme, a 60-heme multiprotein complex containing an octaheme reductase in complex with its redox partner, is now being analyzed.
[1] A. Dietl et al. (2015), Nature 527, 394-397
[2] W. J. Maalcke et al. (2014), Journal of Biological Chemistry 289, 1228-1242
[3] A. Dietl et al.(2015), Acta Crystallographica D71, 1708-1713
[4] W. J. Maalcke et al. (2016), The Journal of Biological Chemistry 291, 17077-17092

