Introduction to Muscle Contraction, Part 2
For ATP-hydrolysis the 50k upper/lower domain cleft should close
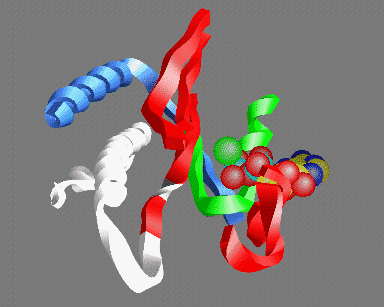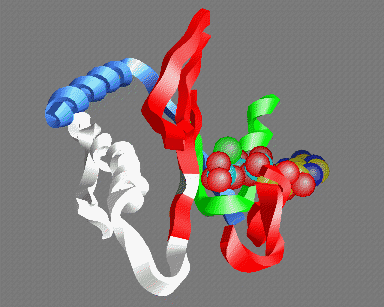
© Kenneth C. Holmes
Fig 5: A view of the ATP binding site looking out from the actin helix. Shown are: the P-loop (green); an MgATP molecule with the base at the back and the three phosphate groups in the front (carbon yellow, nitrogen blue, phosphate light-blue, oxygen red, magnesium green); parts of the 50K upper domain (red) including the so called "switch-1" region (right); the switch-2 region in the open "ATP" (white) and closed "ADP" (gray) conformations - the conserved glycine (gg466) is shown in gray or white- note that this residue moves about 5Å between the two conformations; the helix (gg648-666) (which acts as fulcrum for the relative rotation of the 50K upper and lower domains (blue)
© Kenneth C. Holmes
Smith and Rayment (Smith and Rayment 1996) point out the similarity of the active site of myosin in the closed form with ras p21 and other G-proteins and that the enzymes probably share a common mechanism for hydrolysis (Schweins et al. 1995). The differences between the open and closed forms of the myosin cross-bridge in the neighborhood of the active site reside almost entirely in the conformation of the linker region (gg465-470) which joins the 50K upper and lower domains. Smith and Rayment point out that this region is structurally equivalent to the so-called switch 2 region in ras p21 with which it also has a very strong sequence homology.
The mutual rotation and closing of the 50k upper/lower domains cleft causes this region to move by about 5Å. In the chicken crystal structure (open), which has no bound nucleotide and should therefore be in the rigor conformation, the switch 2 region is pulled away from the nucleotide binding pocket. A similar movement of the switch-2 region depending on whether di- or tri-nucleotide is bound is also found in the G-proteins. Only in the closed form (ADP.vanadate) can the hydrogen bond between the carbonyl of ggG466 and the γ-phosphate (Fig 5), which is an invariant characteristic of the G-protein active sites, be formed. Because of the importance of ggG466 (and other residues) for hydrolysis it is difficult to see how hydrolysis can proceed in the open (rigor) form which would therefore appear not to be an MgATPase: the closing would appear to be essential for hydrolysis.
