Biotin Biosynthesis
P450BioI performs the oxidative cleavage of a carrier protein bound fatty acid in biotin biosynthesis.
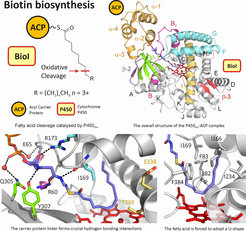
P450BioI (CYP107H1), found in the biotin operon of Bacillus sp., is responsible for the formation of pimelic acid (seven-carbon diacid) via the oxidation of long chain fatty acids. P450BioI performs the selective cleavage of fatty acids between C7 and C8 when the fatty acids are presented by acyl carrier protein (ACP): lack of the carrier protein affords a broad range of hydroxylated fatty acids, with only low yields of pimelic acid. We have structurally characterised several P450BioI-acyl ACP complexes (PDB Codes 3EJB, 3EJD, 3EJE), which reveal the role of the carrier protein in providing a scaffold for oxidation at C7 and C8 in the fatty acid chain. These structures show that the fatty acid chain is accommodated in a hydrophobic active site and is directed across the haem by a “cap” of large hydrophobic residues directly above the haem. The methyl terminus of the fatty acid projects into a large pocket, enabling various length acyl-ACPs to act as substrates. The correct positioning of the chain relative to the haem iron is achieved through interactions between the carrier protein linker and residues of P450BioI, including interactions with both linker amide groups. Towards the protein surface, additional water-mediated and direct hydrogen bonding interactions are found between ACP residues and surface residues of P450BioI.
The P450BioI system represents an important model system for studying carrier protein/P450 interactions, with the mechanism of fatty acid cleavage an additional source of research interest in this system. We are currently undertaking further structural, biochemical and chemical characterisation of the P450BioI-ACP system in collaboration with the group of Prof. James De Voss (University of Queensland, Australia).
