Glycopeptide Biosynthetic P450s
P450-catalysed oxidative coupling of aromatic residues in the biosynthesis of glycopeptide antibiotics: a crucial step in biosynthesis.
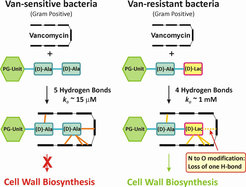
Glycopeptide antibiotics, of which vancomycin and teicoplanin are representative members, are heptapeptide antibiotics produced by certain species of Streptomyces bacteria. These remain effective clinical antibiotics with activity against Gram-positive bacteria, although resistance is increasing. These antibiotics function through the formation of a hydrogen bond-mediated complex with a bacterial cell wall precursor peptide terminating in D-Ala-D-Ala, a complex that consists of five hydrogen bonds involving the glycopeptide amide backbone.
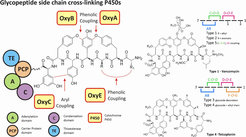
The phenolic coupling of aromatic residues is essential for the activity of these glycopeptides, as these cross links generate the required three dimensional structure needed for these peptides to act as antibiotics. The formation of these phenolic cross links has been shown to be catalyzed by three or four P450 (Oxy) enzymes, respectively. These P450s require the peptide substrate to still be bound to the carrier protein domain of the non-ribosomal peptide synthetase machinery and exhibit at most limited activity with free peptides as substrates.
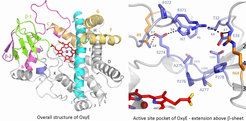
Due to the crucial role that these P450s play in vancomycin biosynthesis, we are undertaking the structural and biochemical characterisation of these P450-carrier protein complexes, with the eventual aim of using these P450s as biocatalysts in a biomimetic synthesis of vancomycin. Through such an approach, the generation of novel glycopeptide scaffolds for screening against resistant bacteria would be possible, an approach that is currently difficult through classical organic synthesis. As an example of recent results, we have structurally characterised and begun the biochemical characterisation of OxyE (CYP165D3) from Teicoplanin biosynthesis. This has revealed modifications to the active site of the enzyme compared to other Oxy structures and has shown a dramatically lower substrate affinity for non-crosslinked carrier protein bound peptides for this enzyme, supporting the serial action of the Oxy proteins.


