Gene Regulation in the brain
Chemically-controlled gene expression in the brain
Initial studies in the mouse showed that Tet-controlled expression systems can be used to study the involvement of genes in learning and memory. However, tTA-dependent reactivation of Tet regulated genes in CNS neurons can be difficult to achieve if their expression was suppressed prenatally; reactivation was very slow and expression patterns were mosaic.
We investigated this problem and found neither lack of transactivator nor access of the tetracycline compound for the brain are responsible for this (Zhu et al., 2007) because neither with high concentrations of transactivator, delivered by AAV infection (left panel), nor by the direct injection of the tetracycline derivative into reverse transactivator-expressing brains could responder genes be activated. We have thus discovered that stably-integrated Ptet can become epigenetically silenced in the majority of neurons when they are transcriptionally inactive during development. Furthermore, epigenetic silencing of the Ptet in neurons does not occur when it is present in a non-integrated state (right panel).
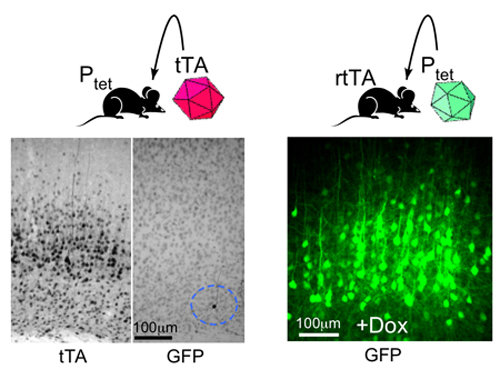
We have also found that in the presence of high tTA levels, epigenetically silent Ptet becomes reactivated in an increasing number of neurons after a prolonged time period (2.5 months). The reversal of gene silencing by high levels of tTA strongly favors chromatin reprogramming as the likely epigenetic mechanism for gene re-activation.
Combinatorial genetic approach for cell-type-specific reversible control of gene expression
We have generated recombinant adeno-associated viruses equipped with the tetracycline-controlled elements for reversible control of gene expression in neurons (Zhu et al., 2007). Moreover, by introducing Ptet responder genes via AAVs into tTA/rtTA transgenic mice, we can achieve cell-type specific expression (see right panel). Our combinatorial genetic approach harnesses the best features of the two methods: achieving Ptet responder gene activation in neurons via AAV-based gene delivery and cell type specific tTA/rtTA expression via transgenesis.
Future directions
Epigenetic regulation of neuronal gene expression is now recognized as on possible mechanism of adaptive cell response to external stimuli. Thus challenging environmental conditions in various biological processes, including stress, addiction, memory functions and diseases can lead to chromatin remodeling by changes in DNA and histone acetylation and histone methylation patterns which lead to changes in gene expression of the modified genes.
Our systematic investigation Ptet silencing phenomenon in neurons provide us with a unique opportunity to (a) systematically investigate the molecular mechanism(s) of epigenetic silencing using the tetracycline-inducible system as a model and (b) re-engineer elements of the tetracycline genetic systems for tetracycline-controlled, rtTA-dependent gene expression in the brain.