Hyaluronan in Lymphangiogenesis
Hyaluronan Interactions
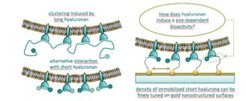
Well-controlled surfaces with immobilized substrates enable novel approaches to investigate specific aspects of biological processes related to cell adhesion or motility. We report a method to prepare self-assembled monolayers on gold surfaces, co-presenting the cell adhesive RGD motif and small hyaluronan molecules, to investigate for example protein[1], integrin containing proteoliposome binding[2] or cell[3] binding.
This technique enables an independent adjustment of the RGD motif and hyaluronan density while maintaining a passivating background. In these studies, the main question was how co-presentation of hyaluronan, an essential component of the extracellular matrix, and the RGD motif affect protein, lymph endothelial cell (LEC) and integrin binding. We develop two strategies for covalent attachment of sHA, a fast high-density adsorption and a two-layer system that allows tuning the density and mode of immobilization. We monitored the sHA adlayer formation and subsequent macromolecular interactions by label-free quartz crystal microbalance with dissipation (QCM-D). The modified surfaces are inert to unspecific protein adsorption, and yet retain the specific binding capacity of sHA. Thus they are an ideal tool to study the interactions of hyaluronan-binding proteins and short hyaluronan molecules as demonstrated by the specific recognition of LYVE-1 and aggrecan. Both hyaladherins recognize sHA and the binding is independent to the presence of the reducing end.LECs grown on the bifunctional adhesive surfaces showed a biphasic change in metabolic activity, with increased metabolic activity being observed in response to increasing nanoparticle densities up to a maximum of 540 particles/μm2. Thus, interfaces that concomitantly present adhesive ligands and sHA can stimulate LEC metabolism and might be able to trigger lymphangiogenesis. A subset of integrins, cellular transmembrane glycoproteins, recognize the evolutionarily conserved tripeptide sequence RGD, and anchor cells to their surrounding proteins as well as mediate bidirectional signaling. Layer formation and subsequent interactions with αIIbβ3 integrins, which are reconstituted in liposomes, was monitored by label-free quartz crystal microbalance with dissipation monitoring (QCM-D). Exceeding a critical RGD motif density of 40% results in enhanced binding of proteoliposomes. Co-presentation studies with varying hyaluronan and constant RGD motif density demonstrate that marginal amounts of hyaluronan are sufficient to prevent integrin binding. These findings are of specific importance in relation to cancer cells, which show highly enriched hyaluronan in the surrounding extracellular matrix to reduce adhesion properties.

[1] Minsky et al. Scientific Reports, 2016, 6:21608.
[2] Zapp et al. Frontiers in Physiology, 2018, 9:1022.
[3] Antoni et al. Frontiers in Bioengineering and Biotechnology, 2018, 6:25.
Mimicking the lymphatic environment
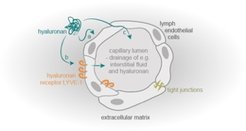
The lymphatic system comprises a network of lymphatic vessels that transports the lymph unidirectionally towards the heart. Lymphatic vessels fulfill three primary roles in humans. (1) The lymphatic vasculature collects interstitial fluid, which leaks from blood vessels in peripheral tissues, and returns them to the blood circulation, to maintain fluid homeostasis. (2) It absorbs dietary fats in the digestive system and transports them back into the blood stream. (3) The lymphatic system, as part of the immune system fights pathogens and initiates the adaptive immune response.
Lymph capillaries are the direct entry point for the lymph and characterized by their direct connection to their surrounding extracellular matrix (ECM). One main component is the glycosaminoglycan hyaluronan (HA), which is built up of repeating disaccharide units. Dependent on its molecular weight and temporal/spatial distribution in the tissue, it has been reported that HA plays a role in numerous biological processes, both alone and through its complex interactions with many HA-binding proteins, matrix components and cells. Therefore, HA contributes among others to the processes of wound healing, tumor progression and metastasis. Moreover, many studies have proven that HA is a critical regulator of angiogenesis. However, little is known about HA ́s role in regulating lymphangiogenesis (formation of new lymph vessels).
Our group works on developing biomaterial systems for shedding light on fundamental issues focusing on cellular interactions with their environment (cell-cell and cell-matrix adhesion) and especially HA, and its influence on higher biological functions. To elucidate the stimulation of endothelial cells (LECs) by HA, a synthetic bottom-up biology approach is chosen. A 3D cell culture system based on functionalized polyethylene glycol hydrogels including HA, adhesion peptides and encapsulated spheroids is established. Preparation of highly reproducible hydrogels with a variable system of parameters enables me to investigate the influence of mechanics (elasticity, mesh size, shear forces...) and chemical cues (varying adhesive motifs and HA) on the behavior and cell signaling of LECs.
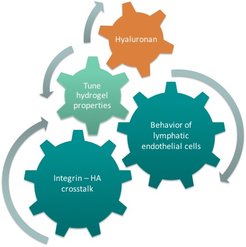
With this interdisciplinary approach, the gap between 2D model systems and in vivo models of lymphangiogenesis will be bridged. Above all, this model can be applied to identify regulators of lymphangiogenesis and to understand a variety of diseases associated with abnormal LEC growth.



