To make a virus - Assembly, DNA loading and processing of virophage capsomers and capsids
Forschungsgruppenleiter: Jochen Reinstein
Virophages are small DNA viruses that replicate in unicellular eukaryotes that are co-infected with giant viruses. They recruit the cytosolic reproduction and assembly machinery of the giant virus whose own propagation is then stalled (see also Working group Matthias Fischer/BMM). Up to now 3 different virophages, namely Sputnik, Mavirus and Zamilon were isolated (1-3). They contain about 20 coding regions in a DNA genome of approx. 20 kb. The most conserved components are two capsid proteins, a protease that presumably assists maturation and a FtsK-HerA type ATPase that supposedly is responsible for ATP dependent DNA transport into the assembled capsid.
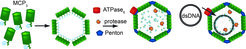
We characterize key features of Mavirus capsid assembly and processing by the respective protease in vitro and in vivo. In continuation with previous studies about protein folding and folding assistance through molecular chaperones, the folding and assembly/disassembly pathways of viral capsid proteins (MCP and penton) are characterized with spectroscopic (light scatter techniques, Fluorescence (also FRET (4)), CD) and structural methods (X-Ray, EM) with the aim to improve our knowledge on the hierarchy of supramolecular organisation of the virophage capsid. This also includes molecular insights into function and structure of the DNA packaging process which is supposed to be mediated by a FtsK-HerA type hexameric ATPase motor protein (similar to the AAA+ ClpB chaperone (5)) and processing of the capsid by the viral Cys-protease (6).
An extension to these in vitro studies will be the use of in vitro assembled and loaded capsids (VLP = Virus like particles) as nano-containers and agents to study the infection pathway in the host Cafeteria through high resolution microscopy (in collaboration with Matthias Fischer/BMM). This project will include the development of new labelling methods to allow detection through fluorescence techniques in vitro and in vivo.
References:
[1] Zhang X, Sun S, Xiang Y, Wong J, Klose T, Raoult D, et al. Structure of Sputnik, a virophage, at 3.5-A resolution. Proc Natl Acad Sci U S A. 2012;109:18431-6.
[2] Fischer MG, Suttle CA. A virophage at the origin of large DNA transposons. Science. 2011;332:231-4.
[3] Gaia M, Benamar S, Boughalmi M, Pagnier I, Croce O, Colson P, et al. Zamilon, a Novel Virophage with Mimiviridae Host Specificity. Plos One. 2014;9.
[4] Muller B, Anders M, Reinstein J. In vitro analysis of human immunodeficiency virus particle dissociation: gag proteolytic processing influences dissociation kinetics. PLoS One. 2014;9:e99504.
[5] Zeymer C, Fischer S, Reinstein J. trans-Acting arginine residues in the AAA+ chaperone ClpB allosterically regulate the activity through inter- and intradomain communication. J Biol Chem. 2014;289:32965-76.
[6] Born D, Reuter L, Mersdorf U, Mueller M, Fischer MG, Meinhart A, Reinstein, J. Capsid protein structure, self-assembly, and processing reveal morphogenesis of the marine virophage mavirus. Proc Natl Acad Sci U S A. 2018;115:7332-7.

