A virus as a symbiosis partner
The mavirus provides single-celled organisms with immunity against a giant virus
All organisms ─ big or small ─ are under constant attack by a multitude of viruses. As a consequence, a variety of innate and adaptive immune defence systems have evolved to keep parasites under control. Researchers at the Max Planck Institute for Medical Research in Heidelberg have now discovered a new type of antiviral defence mechanism in a single-celled eukaryote by which one virus conveys adaptive immunity to the host against another virus, albeit with an interesting twist.
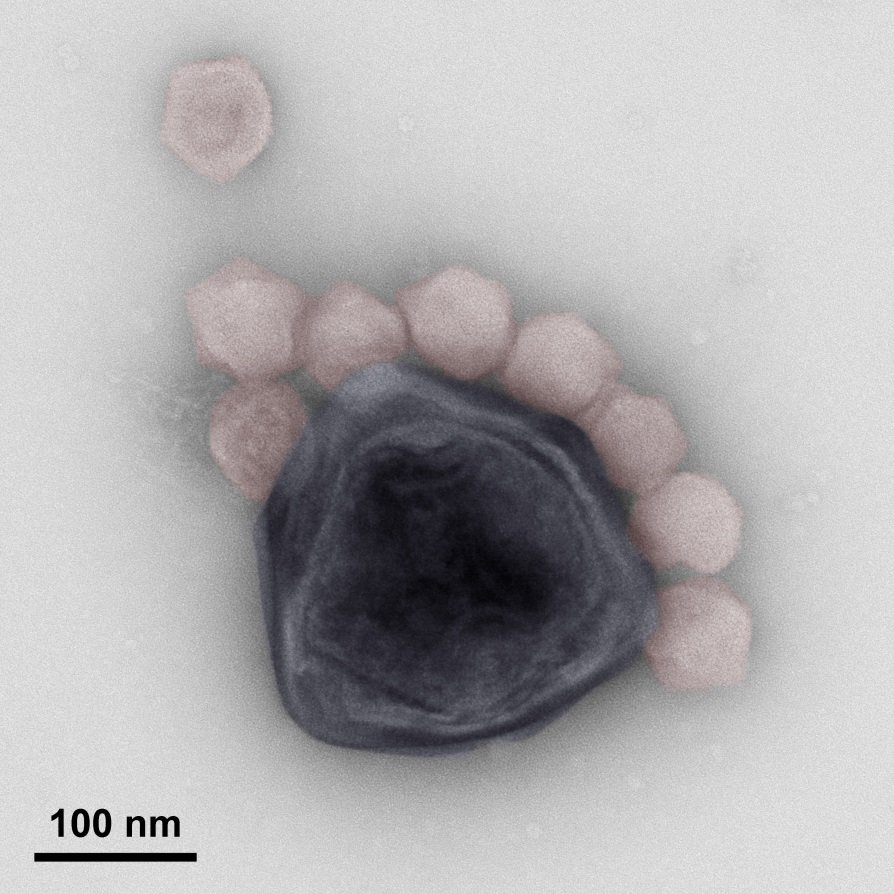
The flagellate Cafeteria roenbergensis is a protist that is found in all the world's oceans where it feeds mostly on bacteria and thus plays a key role in the marine food web. C. roenbergensis and related species can be infected by a giant virus called CroV, which ultimately kills the host cell. Giant viruses encode hundreds of proteins and create complex cytoplasmic replication sites called 'viral factories' that also contain enzymes, such as RNA polymerases, that are normally locked away in the nucleus. This enzymatic complexity in turn attracts smaller viruses called virophages, which parasitize the giant virus factory inside the shared host cell. A virophage named mavirus (for Maverick-related virus) can effectively inhibit the replication of CroV, which results in the release of only mavirus particles, but no new CroV particles, when a co-infected cell lyses. Following the old proverb "the enemy of my enemy is my friend", a population of C. roenbergensis cells may thus survive an infection with CroV in the presence of mavirus.
Protection only in case of simultaneous infection
In a recent paper published in Nature, Matthias Fischer and Thomas Hackl shed some light on the phenomenon of hyperparasitism in this tripartite virus-virophage-host system. They showed that mavirus efficiently inserts its genome into the host cell genome, a process called DNA integration. In its integrated state, the mavirus genes are silent, i.e. no mavirus proteins are produced. However, when CroV infects a cell that contains an integrated mavirus genome, the mavirus genes become active and new virophage particles are produced. Fischer and Hackl observed that in these initially infected cells CroV replication is not inhibited, which is probably due to a slower mavirus response than during a co-infection. As the cells lyse, both CroV and mavirus particles are released, and now, mavirus can shut down CroV replication during subsequent co-infection events. This prevents the production of further CroV particles and protects the still healthy part of the population from infection by CroV.
Therefore, mavirus-based immunity against the lytic virus CroV works only on the population level, not for the individual cell that becomes infected with CroV. Every CroV-infected cell is destined to die, but by releasing reactivated mavirus particles during lysis, the dying cell altruistically provides a means of defense that can protect other cells from being infected by CroV.
Marvirus is maintained even without a giant virus
"This example also shows that not all viruses are detrimental to their hosts," says Fischer. "Mavirus has established a mutualistic symbiosis with its host. As a virophage, mavirus needs for its replication a host cell that is co-infected with a compatible giant virus. In a CroV-free host cell population, mavirus might become extinct. However, by integrating itself into the host genome, mavirus is passively maintained by the cell and can persist in that state for long periods of time until an encounter with CroV activates the hibernating mavirus. Clearly, the host cell also benefits from the presence of mavirus, since it protects the cell population from death by CroV infection."
The ecological role of this newly discovered defense mechanism is still unclear. "Our study was conducted under controlled laboratory conditions and therefore we cannot evaluate the importance of virophage-based immunity in natural settings," explains Fischer. "But now that we know what to look for, we can screen the genomes of other protists for signs of integrated virophages, which will give us a first estimate of how widespread this anti-viral defence system is in the environment. This is yet another example of the remarkable diversity of biological interactions that can be found among microbes."
MF/HR