Resolving the photoswitching mechanism of a fluorescent protein
Combined use of X-ray free-electron laser and spectroscopy provides new insights
Photoswitchable fluorescent proteins are used as molecular markers in super-resolution light microscopy that allows to image living biological cells at a resolution of a few tens of nanometers. These proteins can be reversibly toggled between a non-fluorescent (off) state and a fluorescent (on) state by irradiation with light at specific wavelengths. Photoswitching between on and off states involves ultra-fast excited-state processes that have been recently characterized structurally. Conformational changes on the slower time scale, however, have remained elusive, hampering a comprehensive description of the photoswitching mechanism at the molecular level.
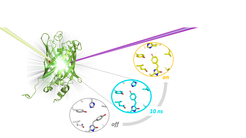
Using time-resolved serial crystallography at the X-ray free-electron laser (XFEL) SACLA in Japan, in combination with transient absorption spectroscopy, researchers from the Department of Biomolecular Mechanisms at the Max-Planck Institute for Medical Research in Heidelberg and collaborators have now established the photoswitching mechanism of rsEGFP2. They determined the three-dimensional structure of a key photo-intermediate which is populated 10 nanoseconds after photoexcitation of the off state (see figure). This study clarifies the order of events during the off-to-on photoswitching and is anticipated to facilitate rational improvement of reversibly photoswitchable fluorescent proteins for applications in super-resolution light microscopy of biological cells.
