On the trail of an old acquaintance
First biosensor for real-time determination of cellular coenzyme A concentrations
The Department of Chemical Biology at the MPI for Medical Research has developed a biosensor that allows for the first time the tracking of concentration changes of the cofactor coenzyme A (CoA) in living cells and the determination of absolute cellular CoA concentrations in real time. The work was recently published in the journal Nature Chemical Biology.
Coenzyme A (CoA) is a cofactor of great importance for cellular metabolism. Cofactors are non-proteinogenic substances, organic compounds that are essential for the catalytic activity of a variety of enzymes. CoA and its acetylated form, acetyl-CoA ("activated acetic acid") are needed for central processes in cellular metabolism, such as the citric acid cycle, cholesterol biosynthesis, amino acid metabolism and, last but not least, fatty acid metabolism. CoA in its acylated form is also of particular importance in the regulation of cellular processes as a substrate for acylation, an important regulatory post-modification of proteins.
Historically, research into coenzyme A has been closely linked to the Max Planck Institute for Medical Research in Heidelberg. Theodor Wieland, director at the institute from 1967 to 1981, investigated the structure and biogenesis of vitamin B5 during his doctoral work under former director and Nobel Prize winner Richard Kuhn. Vitamin B5, also known as pantothenic acid, is an important starting point for CoA biosynthesis. Fritz Lipmann, a former doctoral student of the co-founder of the Institute for Medical Research, Otto Meyerhof, and himself active at the Institute (founded in 1930), was awarded the Nobel Prize for Medicine in 1953 for the isolation and discovery of CoA. The isolation and structural elucidation of acetyl-CoA was achieved by Wieland's brother-in-law and later Nobel Prize winner Feodor Lynen.
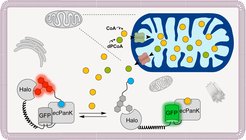
A team from the Department of Chemical Biology at the Max Planck Institute for Medical Research, led by Kai Johnsson and Lin Xue, has now taken on the challenge of unlocking the secrets of this old acquaintance, CoA, directly in the cell. Due to the central importance of CoA for the cell, it is not surprising that dysregulations of CoA concentration are associated with various diseases. To study the cellular regulation of CoA levels and its pathophysiological significance, the concentration of CoA needs to be readable at different time points and within different cellular compartments. The Snifit concept, developed by Kai Johnsson, was used to develop a biosensor that enables such CoA determination within the cell. Snifits are sensor proteins that contain a binding protein for the analyte, a self-labelling protein (SLP) and a fluorescent protein. The SLP is selectively labelled with a fluorescent ligand. The binding of the analyte (here CoA) to the sensor results in a change in the color of the emitted light (e.g. red to green) and the analyte concentration can be read out by simply determining the colour of the emitted light.
Conceptually, designing a Snifit for any analyte sounds quite simple. The difficulty, however, lies in the selection of the individual components of the Snifit. Snifits exploit the displacement of a synthetic ligand from a binding protein by the analyte of interest; how should the appropriate binding protein and ligand pair be chosen? Which arrangement of components in the Snifit results in an easily readable signal? Lin Xue, post-doc in the Department and first author of the study published in Nature Chemical Biology, was able to solve all these difficulties and develop a CoA-Snifit. However, some detailed work was needed to get the final CoA-Snifit. "Enabling the sensor to distinguish between CoA and its acetylated form, acetyl-CoA, was a particular challenge that we were able to solve by introducing targeted mutations in the binding domain of the sensor", says Lin Xue. With the newly developed CoA-Snifit, the team was able to determine CoA concentration in mammalian cells and identify key enzymes that play a central role in the regulation of CoA concentration. With the development of biosensors as a tool for the in situ determination of metabolite concentrations in the cell, Director Kai Johnson's department is following Feodor Lynen's credo: "Nature is always unpredictable, and the only way to pick up a biochemical problem is to do experiments". With the new CoA-Snifit, it is now possible to conduct these experiments directly in cells. The department expects the biosensor to find use as a tool to study CoA and its pathophysiologies in many laboratories worldwide.
