
Equipment
My group is interested in the biophysical characterization of peptides and proteins (and their associated cofactors) and we work in close cooperation with all of the reaerch groups in our department; collaborations with other groups in the institute are starting.
Detailed analyses of proteins are genrally performed via mass spectrometry, which is often coupled with optical spectroscopy. Our equipment includes 3 mass spectrometers.
Shimadzu 2010A single quadrupole mass spectrometer
Shimadzu 2010A single quadrupole mass spectrometer with electrospray (ESI) and athmospheric chemical ionization ion sources.
The 2010A ESI machine is particularly useful for the analysis of metabolites, cofactors, peptides and smaller proteins, up to a mass of about 40.000 DA (see Figure 1). The instrument has also proven useful for monitoring HPLC effluents (LCMS) and in quality control on the integrity of precious nucleotides and neuroactive pharmaceuticals (for the Department of Molecular Neurobiology).
Figure 1
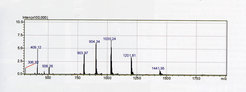
© Yvonne Grömping
ESI spectrum of a recombinant intersectin SH3 domain-containing protein (Yvonne Grömping Group, Dep. of Biomolecular Mechanisms). The protein exhibits multiple peaks, with charges from +5 to +9 and thus a range of mass/charge (m/z) values. The experimentally determined mass of the intact protein is 7204.6 Da, which is in good agreement with the calculated average mass of 7205.4 Da.
© Yvonne Grömping
The 2010A ESI machine is particularly useful for the analysis of metabolites, cofactors, peptides and smaller ESI spectrum of a recombinant intersectin SH3 domain-containing protein (Yvonne Grömping Group, Dep. of Biomolecular Mechanisms). The protein exhibits multiple peaks, with charges from +5 to +9 and thus a range of mass/charge (m/z) values. The experimentally determined mass of the intact protein is 7204.6 Da, which is in good agreement with the calculated average mass of 7205.4 Da.
