Collective Cell Migration
Collective cell migration is the process of several cells migrating as a cohesive group, in which each individual actively coordinates its own movement with that of its neighbors. It plays very critical roles during embryogenesis, wound healing, and cancer progression. We are taking an interdisciplinary approach to understand how physical forces and stresses at the single cell and multicellular levels interact with the biochemical signaling pathways to support the well-orchestrated movement of several cells in moving epithelial monolayers.
Mechanobiology of Collective Migration
Many organisms, including us, often move in groups. In such moving groups or ‘collectives’, every individual consciously tries to align its movement along that of its neighbors. This process requires well orchestrated sensing and action, which are mutually connected through the signal transduction within the nervous system. At cellular level, collective movement emerges literally out of the tug-of-war among the cells, leading to large-scale fluctuations in cell-substrate traction force landscape. However, from the fluctuations, it is also possible to isolate a systemic behavior and find a relationship between the forces and the movements. Here, we are trying to reveal the molecular mechanisms that give rise to this relationship (Das et al. Nature Cell Biology 2015). To this end, we are specifically interested in understanding how the forces and the stresses in a epithelial monolayer control the molecular and structural polarization of cells, which are correlated in multicellular length-scale.
Collaborating partner: Jeffrey Fredberg, Harvard University
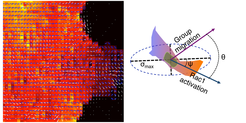
Leader Cells
During collective migration of epithelial cells, few cells in the wound margin, about one in every five, show prominent lamellipodial protrusions. These cells are known as the ‘Leader cells’ as they control the directionality and the persistence of the group movement. However, we know very little about the underlying molecular mechanism of the leader cell generation. Especially, it remains puzzling why among all cells in the wound margin only few undergo this drastic transformation to become leader cells. We are currently taking a combined biophysical and molecular biology approach to attain a systemic understanding of the leader cell generation and its regulation.
Collaborating partner: AG Boehm, MPI for Medical Research

Confinement Effect
Cells in our body mostly reside within the confined environment of tissue matrices and circulatory vessels. These micron-sized confinements or ‘microconfinements’ influence embryonic morphogenesis, tissue regeneration, and most importantly, cancer progression. Within microconfinement, while at one end, cellular signaling intensifies due to restricted diffusion and local build-up of morphogen concentration, at the other, the mechanical cues of the microenvironment significantly influences the cell growth and migration dynamics. Also the dynamics of cell movements in a very confined system, when they are sandwiched between two surfaces, can be qualitatively different from the dynamics of the conventional 2D migration. In a sandwich-system both surfaces can present extracellular matrix (ECM) or cell adhesion proteins. Though there have been some progresses recently in understanding how confinement affects the single cell migration, its effect on collective migration remains elusive. We are now trying to mechanically dissect the effect of microconfinement on collective migration of epithelial cells.
Collaborating partner: Christian Franck, Brown University



