Focal Adhesion Dynamics
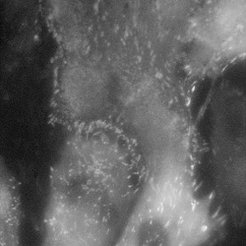
Fibroblast cells (REF) stained with YFP paxillin
Normal mode
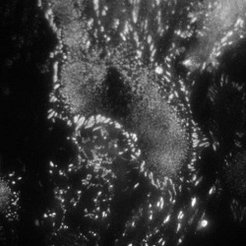
Fibroblast cells (REF) stained with YFP paxillin
TIRF mode
Using single molecule microscopy, we study the diffusive behaviour of proteins in living cells. Special emphasis is put on the influence of nanostructures on the protein diffusivity. The attachment of cells to the extracellular matrix is governed by transmembrane proteins called integrins. These proteins form organized attachment structures, the so-called focal adhesions. The stability of focal adhesion contacts depends crucially on the lateral spacing of individual cell adhesion peptides on the nanometre scale. Experiments on precisely tailored nanostructured substrates have shown a threshold spacing of around 60-70 nm to be the critical spacing above which focal adhesions don’t form.
We aim at understanding the physical mechanism responsible for the apparent threshold in stability. This can be achieved by studying the integrin mobility in the cell membrane of fibroblasts (e.g. rat embryonic fibroblasts expressing either GFP-3 integrin or YFP paxillin), especially in and around focal adhesion sites. A basic understanding of integrin clustering and formation of focal adhesions on a molecular scale is crucial for the design of nanostructures which interact in a predictable and controllable way with the cells. This offers powerful solutions in many fields of applications, such as implant technology, tissue engineering, and cancer treatment.

